The raw water which is found in various natural sources can’t be directly used by the public for various purposes, before removing the impurities.
For palatability, the water should be free from unpleasant tastes, and odors and must have a sparkling appearance. It must be free from disease-spreading germs.
The amount & type of treatment process will depend on the quality of raw water & standard of quality to be required after the treatment.
Objective of Water treatment process
- To remove impurities up to the level recommended in water quality standards.
- To kill pathogens harmful to human health.
- To make water safe & potable for drinking.
- To reduce the objectionable color, odour, turbidity & hardness.
- To eliminate the corrosive nature of water affecting pipe.
- To remove dissolved gas, murkiness, etc.
- To remove suspended impurities such as silt, clay, sand, etc.
How many steps are in the water treatment process?
There are about 8 steps in the water treatment process. They are given below.
- Screening
- Plain sedimentation
- Sedimentation with coagulation
- Filtration
- Disinfection
- Aeration
- Softening
- Miscellaneous
Altogether the treatment process can be listed below from the intake of water to the distribution system.
Raw water >>> screening >>> mixing with coagulant >>> Filtration >>> Sedimentation >>> Flocculation >>> Disinfection >>> clear water reservoir >>> service reservoir >>> Distribution
Water treatment process | Water treatment steps
| S.N | Treatment process | Impurity Removal |
| 1 | Screening | Large suspended & floating matters |
| 2 | Plain sedimentation | Coarse & suspended impurities such as silt, clay, sand, etc. |
| 3 | Sedimentation with coagulation | Fine suspended & colloidal impurities. |
| 4 | Filtration | Very fine suspended & colloidal matters, micro-organ |
| 5 | Disinfection | Pathogenic bacteria |
| 6 | Aeration | Taste, odour, dissolve, iron, manganese etc |
| 7 | Softening | Hardness of water |
| 8 | Miscellaneous | Dissolved metals & gases as indicated by water test or analysis |
1) Screening
The process of removing large suspended matter like sticks, branches of tree, leaves, debris, dead animal body, etc before entering through intake using different size screens is called screening.
Purpose of screening
- To remove large suspended & floating matters.
- To increase efficiency of successive treatment plants.
Depending upon the purpose & objectives, screens may be classified as
- Coarse screen
- Medium screen
- Fine screen
1) Coarse screen
Generally, it is placed in front of fine screens at the outlet to remove the large suspended &floating matters.
- It consists of bar grills of 25 mm in diameter.
- The opening is of 50 mm to 150 mm.
- Bars are kept at 300 to 800 inclined with vertical.
2) Medium screen
Generally, placed after the coarse screen to remove medium suspended & floating matters. Opening is of 20-50 mm.
3) Fine screen
It is used to remove small suspended matters, generate this screen is not used because it gets clogged quickly & needs frequent cleaning.
It has a mechanical cleaning device & made of a perforated plate with a diameter of 0.6 cm perforation.
2) Plain sedimentation:-
It is the process in which water is retained in a tank or basin so that the suspended impurities present in the water may settle down under the action of gravity.
The main purpose of plain sedimentation is to remove large amounts of suspended solids present in raw water.
Plain sedimentation is done after screening & before sedimentation with coagulation & located near the filter units & in case of variation of demand, it can be used as the storage reservoir.
Suspended impurities may be,
- Inorganic solids —– sp.gr = 2.65
- Organic solids —— sp.gr = 1-1.4
3) Sedimentation with coagulation:
Very fine suspended impurities/particles such as clay, silt, and light colloidal matter do not settle down in a plain sedimentation tank in a reasonable detention period.
For this, certain chemicals are added to raw water & mixed thoroughly. It causes the formation of an insoluble, gelatinous, flocculent precipitate called floc. This floc becomes heavier & gets settled.
The chemical which is added for the formation of floc is known as coagulation & process of formation of floc is known as flocculation.
The theory is that the ions are +ve changed & attract -ve changed suspended particles of clay, silt & colloidal matter present in water thus the floc becomes heavier & settles down which is further removed by sedimentation.
Coagulants
The chemical added to water for the formation of floc is known as coagulants.
The common coagulants are,
- Aluminium sulphate [Al2 (so4)3.18 H2o]
- Iron salts [feso4.7H2o, fe2(so4)3.fecl3]
- Chlorinated copperas [fecl3.fe2 (so4)3]
- Sodium aluminate [Na2Al2O4]
The dose of coagulants depends upon,
- Turbidity
- Colour
- PH of waterTemperature
- Time of settlement
4) Filtration
The process of passing water through beds of sand or other granular material is known as filtration.
Sedimentation & sedimentation with coagulation removes a large proportion of suspended as well as colloidal particles that have a specific, gravity more than water, but there are particles that have a specific gravity less than or equal to water that can’t be settled in the sedimentation process for removing such particles, filtration is needed.
The purpose of filtration is to remove color, odour, taste, bacteria & colloidal impurities.
Theory of filtration:-
When water is allowed to pass through the bed of filter media, the following action takes place.
- Mechanical straining
- Sedimentation & adsorption
- Biological metabolism
- Electrolytic action
a) Mechanical straining
The particles of suspended matter that are of a size larger than the size of the voids between the sand grains cannot pass through the voids & removed by the action of mechanical straining.
Due to the smaller in size of colloidal matter or bacteria, they may not be strained.
b) Sedimentation & Adsorption
The interstices between the sand grains act as a very smaller sedimentation tank where the suspended matter settles.
Due to physical attraction between the suspended particle & sand grain and the presence of gelatinous coating formed due to these matters, other suspended particle colloidal matters & bacteria adhere there & are removed.
c) Biological metabolism
Organic matter such as algae plankton are also caught be voids between sand gains & this matter is used by bacteria for survival & convert into harmless compounds.
d) Electrolytic action
When water comes in contact with sand particles in filter media having some polarity, the charge is neutralized & chemical characteristics of water change as a result pure water is obtained.
5) Disinfection
The process of killing the infective bacteria from water & making it safe to the user is called disinfection. The chemicals or substances which are used to kill the bacteria are known as disinfectants.
Disinfection is the most important step in the treatment process as it removes hazardous bacteria which is more harmful than turbidity, colour, odour, etc.
Purpose of disinfection
- To kill organisms & pathogenic bacteria present I water.
- To reduce the chances of epidemics.
- To prevent people from waterborne disease.
Characteristics of a good disinfectant
The disinfectants should be able to destroy all harmful bacteria economically within the contact time & in a wide range of temperatures & PH values.
- It should not render the water toxic or impart colour & odour.
- It should be easily available at a reasonable cost.
- Must be safe to handle & method of application should be simple.
- Disinfection kill only pathogenic bacteria
- Sterilization kills both pathogenic & non-pathogenic bacteria.
Methods of Disinfection
There are various methods of disinfection. They are
- Chlorination
- By boiling of water
- By UV rays
- By the use of iodine and bromine
- By the use of ozone
- By the use of excess lime
- By using potassium per magnet (KMnO4)
- By treatment with silver or electro-kata dan process.
Among the above, chlorination is the most effective & commonly adopted method of disinfection.
a) Chlorination
When chlorine & its compound are used in proper quantity as a disinfectant in water is called chlorination.
Cl2 + H2o ———–> (PH>5) Hocl + Hcl
The hypochlorite acid further dissociates into hydrogen ion (H+) & hypochlorite ion (Ocl–)
Hocl ———–> (PH>8) H+ + ocl–
The undissociated Hocl is about 80 – 100 times more destructive than ocl– . Hocl is highly unstable & rapidly decomposes on exposure to sunlight.
Hocl + ocl + cl2 ———–> Combinely called free chlorine.
The amount of chlorine consumed in the oxidation of inorganic & organic matters present in the water is known as the chlorine demand of water.
The dose of chlorine is the required amount of chlorine to be added which leaves about 0.2mg/L after a contact period of 10 minutes.
Chlorine dose = Chlorine demand + residual chlorine.
The amount of chlorine remaining in water as unreacted is known as residual chlorine.
6) Aeration
The method of bringing the water into contact with atmospheric air to increase dissolved oxygen in water is called aeration.
The purpose of aeration are:-
- It removes taste & odour from water.
- Helps in the mixing of chemicals in the water.
- To precipitate iron & manganese present in water by converting soluble state.
- To reduce the corrosiveness of water.
- To make water fresh.
- To release dissolved gas (Co2, H2s) to the atmosphere.
- To destroy micro-organisms.
Method of aeration
- Free fall or gravity aeration
- Cascade aerator
- Inclined aerator
- Salt tray aerator
- Gravel-packed bed aerator
- Trickling bed aerator.
- Spray aerator.
- Diffuse air aerator
- Mechanical aerator
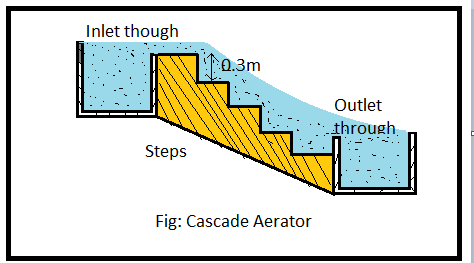
a) Cascade aerator
In this method, the water is allowed to fall over a series of concrete steps. During the fall, the water gets thoroughly mixed with the atmospheric all & gets aerated.
b) Inclined Aerator
In this type of aerator, water is allowed to fall in inclined plains with riffle plates.

Note:- Generally aeration is done for groundwater due to the low oxygen contained in it.
c) Salt tray aerator
Consists of closely stacked superimposed wood salt trays.
Water is sprayed evenly on the top tray.
Water trickles from one tray to other.
Air is supplied at the bottom with the help of a blower. The ventilator discharges air & gases.

d) Gravel packed bed aerator

In this aerator, water is allowed to fall through beds of coke, lime-stone, or anthracite & air is blown from the bottom. Co2 can be removed efficiently from water by using such type of aerator.
e) Trickling bed Aerator
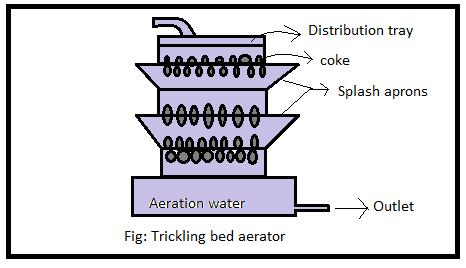
It is the form of gravel bed aerator & is commonly used as aeration to treat groundwater.
Three or four trays having perforated bottoms filled with coke, slag, or stone are used.
f) Spray Aerator:-

In this aerator, water flow is divided into fine streaming & small droplets which come into contact air & aeration takes place. In this method, water is sprayed to the atmosphere through a nozzle at a pressure of 0.70 to 1.50 kg/cm2 for aeration. It can remove 70 – 90% Co2.
g) Diffuse air aerator:-

In this aerator, a perforated pipe network is installed at the bottom of the aeration tank & compressed air is blown through these pipe networks as shown in the figure.
The air bubble travels upward through water causing aeration. The aeration tank has a depth of 3-5m & retention periods of 15 minutes.
h) Mechanical Aerator
In this method, aeration is done in a tank with a mechanical rotating device that mixes air with water.

7) Miscellaneous treatment
The impurities present in water cannot be removed completely by the processes of coagulation, sedimentation filtration, disinfection, and softening. So, certain methods of water treatment that are required for a specific purpose are called miscellaneous treatments.
The method of miscellaneous treatment are:-
- Removal of iron & manganese
- Removal of colour, odour & taste.
A) Removal of Iron & manganese:
Iron & manganese are found in the water in the form of dissolved salts.
The permissible limit of iron & manganese should be 0.3 PPM & 0.1 PPM.
The dissolved iron can be removed by oxidation to the insoluble ferric oxide by simple aeration. The ferric oxide settles down in the form of precipitate in the sedimentation tank & can be easily removed.

When iron & manganese are present in combination with organic matter, then they are removed by adding lime or chlorine, potassium per magnate etc.
B) Removal of colour, odour & taste
The colour, odour & taste in water is due to the presence of dissolved gases such as H2s, organic matter, mineral & microorganisms, industrial waste, etc.
The methods which are used for removal of colour, odour & taste are as follows.
- Aeration
- Treatment with activated carbon
- Treatment with copper sulphate
- BY oxidation of organic matters
1) Aeration
It is a unit process in which air & water are brought into intimate contact.
2) Treatment with activated carbon
Activated carbon is made from lignite, paper mill waste, sawdust & other similar materials by heating at about 500o in close vessels at controlled conditions.
The powdered activated carbon is added either before or after coagulation with sedimentation but used always before filtration.
Activated carbon is also used in granular form as a filter media, instead of using sand in a rapid gravity filter.
It increases the coagulation power of the process, reduces chlorine demon & removes colour, odour & taste.
3) Treatment with copper sulphate
The normal dose of cuso4 is from 0.5 to 0.66 ppm in treated water.
The advantage of copper sulphate is that it checks the growth of algae even before its production & kills some bacteria.
The disadvantage of copper sulfate in excessive doses is to kill fish & other water-living creatures.
4) By oxidation of organic matters
Chlorine oxide gas & ozone can be used for oxide purposes but due to their heavy cost, they are uneconomical.
Chlorine, potassium per magnate, ozone, etc are the oxidizing agents which are commonly used.
Happy Learning – Civil Concept
Read Also,
Types of Impurities in water | Quality of Water – Water Supply
Types of water demand | Formula to Calculate